There are three physical states of matter: solid, liquid, and gas. Under specific temperature and pressure conditions, these states can be used interchangeable. A change in state of matter is the process of changing from one state to another.
Changes in the energy levels and intermolecular forces of the particles within a material cause this transition. Since they explain a wide range of industrial and natural phenomena.
Change of State of Matter
Latent heat: It s defined as amount heat required to change the state of unit mass of a substance at a constant temperature.
The latent heat of a substance is given by L = Q / m where m is mass of a substance.
The SI unit of latent heat is J kg-1 where as practical unit is
cal g-1.
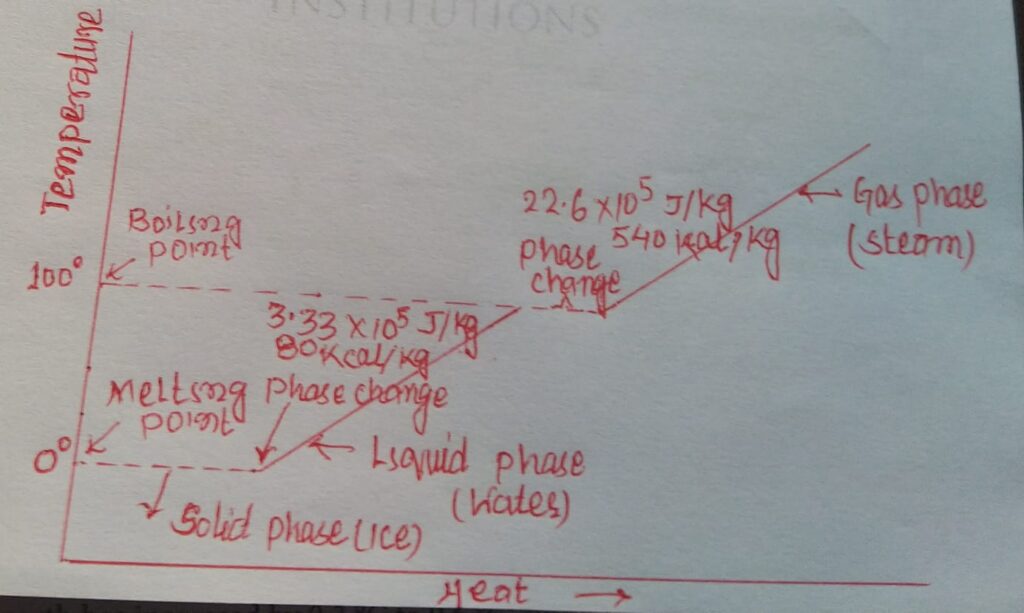
Latent heat of fusion: It is the amount of heat required to change unit mass of the solid into liquid at constant temperature, e.g. latent heat of fusion of ice = 80 cal g-1.
Latent heat of vapourisation: It is the amount of heat required to change unit mass of liquid into vapour at constant temperature e.g; latent heat of vapourisation of water = 540 cal g-1.

If we draw a graph between temperature (T 0C) and heat energy for a quantity of ice converted into steam, we shall get a curve as shown here.
The temperature and pressure at which all the three phases of water exist in equilibrium is called triple point of water.
Triple point of water = 6.11 x 102 Pa = 6 x 10-3 atm.
Triple point temperature = 0.010C.
Factors Affecting the Change of State
The following elements have an impact on the change in matter’s state:
Temperature: Particle kinetic energy is influenced by temperature changes, which result in state changes.
Pressure: A change in state might result from applying pressure, which can push particles closer together or permit them to move away.
Intermolecular Forces: The ease with which a substance can alter its condition is determined by the intensity of the attractive forces that exist between molecules.
Types of Changes in the State of Matter
Melting (Fusion)
The process by which heat transforms a solid into a liquid is called melting. The melting point is the temperature at which this happens. The particles acquire enough energy at this temperature to break away from their fixed locations and begin to move more freely.
Example: Ice melts into water at 0°C.
Freezing (Solidification)
The opposite of melting, freezing occurs when heat is removed from a liquid, causing it to solidify. The freezing point, also known as the melting point for a certain substance, is the temperature at which freezing takes place.
Example: Water freezes into ice at 0°C.
Boiling and Evaporation
The process by which a liquid turns into a gas at a specific temperature known as the boiling point is call. On the other hand, evaporation happens at any temperature below the boiling point when surface molecules acquire sufficient energy to transition into the gaseous state.
Example: Water boils at 100°C under normal atmospheric pressure.
Condensation
The process by which a gas cools and becomes a liquid is called condensation. When gas molecules lose energy and approach one another to create a liquid, this occurs.
Example: Water vapor in the air condenses into droplets on a cold surface.
Sublimation
The immediate transformation of a solid into a gas without first going through the liquid state is known as sublimation. This happens in materials where particles can escape straight into the gas phase due to high intermolecular interactions.
Example: Dry ice (solid CO2) sublimes directly into carbon dioxide gas.
Deposition
The opposite of sublimation, deposition occurs when a gas turns straight into a solid without first producing a liquid.
Example: Frost formation from water vapor in cold conditions.
Energy Changes in State Transitions
Each change of state are an energy exchange:
Endothermic Processes (absorb heat): Melting, Boiling, Sublimation
Exothermic Processes (release heat): Freezing, Condensation, Deposition
The temperature doesn’t change throughout these transitions until the entire substance has changed to the new state.
Applications of Change of State
There are several uses for the state change concepts in both daily life and business:
Air conditioning and refrigeration: Cool areas by using the condensation and evaporation of refrigerants.
Cooking: Two important steps in food preparation are boiling and evaporation.
Dry Ice: Used for sublimation cooling and fogging effects.
Meteorological Phenomena: Deposition and condensation lead to the production of clouds, fog, and dew.
