The areas of an atom with the best chance of containing an electron are known as atomic orbitals. These orbitals offer a basic understanding of the distribution of electrons around an atomic nucleus and are mathematical functions that are obtained from the Schrödinger equation. Quantum numbers give each orbital its different shape, which affects the atom’s physical characteristics and chemical bonding.
Quantum Numbers and Orbital Shapes
Quantum numbers parameters that specify the energy levels and spatial distributions of electrons within an atom are vital to understand the forms of atomic orbitals. These are the four categories of quantum numbers.
1.Principal Quantum Number (n) – Establishes the electron’s energy level and calculates the orbital’s size. The orbital extends farther from the nucleus when n is higher.
2. Azimuthal Quantum Number (l) – Determines the shape of the orbital. For a given n, l can take values from 0 to n−1, with each value representing a specific orbital type:
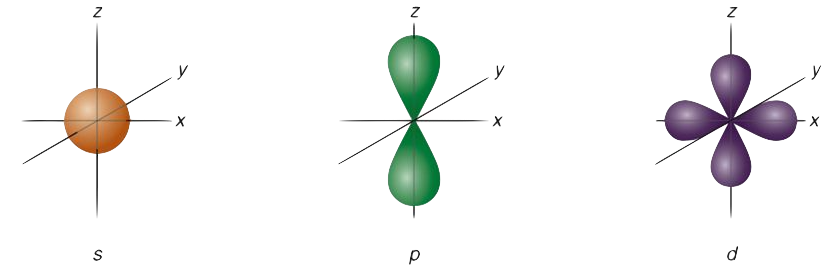
l=0: s-orbital (spherical shape)
l=1: p-orbital (dumbbell shape)
l=2: d-orbital (complex, cloverleaf shapes)
l=3: f-orbital (even more complex shapes)
3. Magnetic Quantum Number (ml) – Specifies the orientation of the orbital in space. It can take values from −l to + l.
4. Spin Quantum Number (ms) – Specifies the spin direction of the electron within the orbital, either -1 / 2 or +1 / 2.
Types and Shapes of Atomic Orbitals
1.s-Orbitals (Spherical Shape)
s-orbitals are the most straightforward and symmetrical. Because of their spherical shape, the electron density is dispersed uniformly across the nucleus.
This spherical form is isotropic, means that the distribution of electron density has no preferred direction. Higher energy levels are indicated by larger orbitals, as an s-orbital grows in size as n values increase. The 1s orbital, for example, is slightly smaller than the 2s orbital, which is smaller than the 3s orbital.
Atoms like hydrogen that have electrons only in s-orbitals have isotropic qualities, which means that their chemical activity is the same in all directions. This is explained by the spherical form of s-orbitals.
2. p-Orbitals (Dumbbell Shape)
p-orbitals have a unique dumbbell shape, with two lobes extending in opposite directions from the nucleus. The probability distribution is highest along one axis, with the nucleus at the center.
There are three p-orbitals (px, py, pz), corresponding to the ml values of -1, 0, and +1. These orbitals are oriented along the x, y, and z axes, respectively, giving them a specific direction in three-dimensional space.
A key factor in covalent bonding is the direction of p-orbitals. For example, the p-orbitals of carbon atoms overlap with those of other atoms to form pi (π) bonds, which give molecules like benzene and other organic compounds their stiffness and direction.
4. d-Orbitals (Cloverleaf Shape)
The d-orbitals are more complex, with five possible orientations (dxy, dxz, dyz, dx2−y2, and dz2. Most d-orbitals have a cloverleaf shape with four lobes. The dz2 orbital is unique, having a doughnut-like ring around the central lobe along the z-axis.
d-orbitals play a key role in the formation of complicated ions and covalent bonds in transition metals due to their complexity and particular orientations. For example, transition metal complexes such as iron (Fe), copper (Cu), and nickel (Ni) frequently exhibit individual characteristics including color and magnetic behavior due to the geometry of their d-orbitals.
4. f-Orbitals (Complex Shapes)
f-orbitals are the most complicated. They have many lobes that extend in different directions, giving them complex forms. In elements like lanthanides and actinides, the f-orbitals play a significant role in complicated bonding and special characteristics like strong magnetic moments and particular electronic configurations.
In heavy elements, when relativistic effects and complex electron interactions become relevant, the shape and complexity of f-orbitals are especially vital. These orbitals add to the unique properties of rare-earth metals, which find usage in magnets, electronics, and other high-tech fields.
Significance of Orbital Shapes in Chemical Bonding
Chemical bonding is significantly influenced by the shape of atomic orbitals. When atoms go close to one another, their orbitals overlap, and made possible of sharing or transfer of electrons and the creation of bonds. The forms and orientations of the orbitals involved determine the kind and degree of this overlap:
s-Orbitals can overlap in any direction due to their spherical shape, leading to sigma (σ) bonds, which are strong and stable.
p-Orbitals overlap to form sigma bonds (along the internuclear axis) or pi bonds (side-to-side overlap).
d- and f-Orbitals provide additional bonding possibilities, Thats why transition metals can form a variety of complex structures.
Note
Atomic orbitals explain chemical bonding and atomic structure. The wide range of chemical behaviors seen in nature is a result of the different qualities and interactions provided by the spherical s-orbitals, dumbbell-shaped p-orbitals, cloverleaf d-orbitals, and complicated f-orbitals.
The basis for forecasting how atoms will interact, form bonds, and form molecules with particular geometries and features is provided by these shapes and the quantum numbers that define them in chemistry. Impacts our environment, such as organic chemistry, material science, and quantum physics, this information is essential.
The azimuthal quantum number (ℓ) determines the form of an atomic orbital. S-orbitals (ℓ = 0) are spherical, p-orbitals (ℓ = 1) are dumbbell-shaped, d-orbitals (ℓ = 2) are cloverleaf-shaped, and f-orbitals (ℓ = 3) have more complex geometries. These orbital shapes are correlated with different values of ℓ.
Since the electron density is uniformly distributed in all directions surrounding the nucleus, s-orbitals are spherical. The orbital’s wave function, which is solely dependent on its distance from the nucleus and not on its orientation, is what causes this spherical symmetry.
With two lobes on opposite sides of the nucleus, a p-orbital resembles a dumbbell. Each of the three p-orbital types px, py, and pz is positioned along a different axis in three dimensions.
Compared to s- and p-orbitals, d-orbitals have more complex forms. With the exception of the dz2 orbital, which has a distinctive structure resembling a doughnut around a core lobe, they classically have a cloverleaf shape with four lobes. The total number of d-orbitals is five.
The morphologies of f-orbitals are complex, with several lobes projecting in different directions. Because of the higher azimuthal quantum number (ℓ=3), they have more spatial nodes and a higher energy level, making them complex. For elements like actinides and lanthanides, their complex structures are vital.
The way that orbitals can overlap with those of other atoms to create bonds depends on their shapes. For example, s-orbitals’ spherical shape permits uniform overlap, whereas p- and d-orbitals’ directional shapes influence molecule geometry by contributing to certain bonding angles.
Quantum numbers specifically, the principal quantum number (n) and azimuthal quantum number (ℓ) define the size and shape of an orbital. The value of ℓ determines the shape (s, p, d, or f), while n influences the orbital’s size and energy level. Together, they define the spatial distribution of electrons around the nucleus.